Types of Chemical Reactions Worksheet: From Synthesis to Decomposition
Updated:
15 mins read
Do you know the meaning of the term chemical reaction? Well, we will define it for you in a simpler manner. A chemical reaction is a change a substance undergoes to form a new one with different chemical properties. So, in short, substances react to form new substances called products.
For instance, when water evaporates and becomes vapor, it is a chemical reaction. So, the water we had, becomes vapor but now with a different chemical identity.
So, in this article, we will discuss types of chemical reactions worksheet answers, examples of chemical reactions, and our chemical reactions answer key.
How to Identify a Chemical Reaction
Chemical reactions always occur everywhere around us. For instance, if you check outside, you will notice rusted iron sheets or metals. Therefore, it's best to understand how to identify a chemical reaction.
A chemical reaction has the following characteristics;
- Gas evolution
- Color change
- Emission of heat
- Formation of precipitate
- Change of state
- Emission of light
An example of a chemical reaction is respiration, where we inhale oxygen that reacts with glucose to give out carbon dioxide(gas), water, and energy.
Examples Showing the Features of a Chemical Reaction
Let's look at these examples to understand the features of chemical reactions as well as the types of chemical transformations:
Evolution of gas - when a reaction occurs and elements change into a compound and a gas. For instance, zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas.
Zn +2HCL =ZnCl2 +H2
Color change–when some reactions occur, the process changes color. An example is where colorless lead nitrate reacts with potassium iodide to form a yellow precipitate of lead iodide and colorless potassium nitrate.
Pb(NO3)2 +2KI =Pbl2 +2KNO3
Emission of heat - when some reactions occur, there is a temperature change. Those reactions that emit heat are exothermic: they cause a rise in the temperature of their surroundings. In contrast, the reactions that absorb heat from the surroundings are endothermic. An example of an exothermic reaction is combustion, where substances like fuel burn to release heat.
Formation of a precipitate - a precipitate formation occurs when barium chloride reacts with sodium sulphate to form sodium chloride and barium sulphate precipitate.
BaCl2 +Na2SO4 = BaSO4 +NaCl
Change of state – some reactions result in a change of state of the products. For instance, ammonia gas reacts with hydrogen chloride gas to form solid ammonium chloride crystals.
NH3g+ HCLg = NH4 CLs
Types of Chemical Reactions
Several types of chemical reactions occur in our environment. These reactions include;
- Synthesis reaction - another name is the combination
- Decomposition reaction
- Single-replacement reaction
- Double replacement reaction
- Precipitation reaction
- Combustion reaction
- Acid base-neutralization reaction
- Redox reaction
NB: Precipitation and neutralization reactions are double replacements as they result in a completely new compound.
Chemical reactions occur when the starting materials known as reactants differ from the products. As a result, the products may differ in state, color, and more. When reactions occur, they involve the movement of electrons that form and break chemical bonds.
That's why you find that it's easy to break chemical bonds in some reactions, while in others, it's hard. Like in exothermic reactions that release heat, they break chemical bonds easily and thus release energy (in the form of heat) to the surroundings.
Let's discuss the different types of chemical reactions.
1. Synthesis Reaction (combination)
A reaction happens when one or more compounds combine to form a complex compound. An example of this reaction is when hydrogen gas reacts with oxygen to form water.
2H2g +O2 g = 2H2O l
Another illustration of a combination reaction occurs when oxygen reacts with an element, resulting in an oxide. Metals and non-metals react with oxygen, forming an oxide. For example, when you burn magnesium, it reacts with oxygen, producing magnesium oxide. The magnesium powder product is white.
2Mg s +O2 g = 2MgO s
2. Double Replacement Reaction
This reaction is also called metathesis or double displacement reaction. This reaction occurs when aqueous solutions of anions (negatively charged ions) and cations (positively charged ions) react to form an insoluble precipitate. As a result, the products have insoluble solids (precipitate) and the remaining liquid called supernate.
Again, double replacement still means when positive and negative ions in two ionic compounds trade for places to form two new compounds.
For instance, when AB + CD = AD+ CB. A and C are positive ions, while Band D is negative. So, in double replacement, A (positively charged) combines with D (negatively charged) and C with B, exchanging places to form new products. Double displacement reaction examples include adding a few drops of lead(II) nitrate to a potassium iodide solution. The results are lead (II) iodide which is a yellow precipitate.
3. Decomposition Reaction
This reaction involves the breakdown of a compound into two or simpler substances. The formula of decomposition is as follows;
AB = A+B
academic performance and reach your goals
See how quick and easy it is to get an exceptional essay with minimal effort on our platform.

For decomposition reactions to occur, they require energy to break down the bonds, either light, heat, or electricity. A simple example of a decomposition reaction involves binary compounds: composed of two elements. Decomposition can be both endothermic and exothermic depending on the compounds involved.
a). Another one is the decomposition of carbonic acid in soft drinks.
H2CO3 = H2O + CO2
b). Electrolysis of water to give hydrogen and oxygen
2H2O = 2H2 + O2
c). The decomposition of Ozone (O3) to give oxygen is exothermic, and it's known as photochemical decomposition: where reactants break down into products using photon's energy.
O3 + hv = O2 = O
4. Single Replacement Reactions
This reaction occurs when a single element replaces a similar element in a compound. It's still referred to as a single displacement reaction or substitution. A simple formula of the reaction includes;
A + BC = AC + B – in this case, element A, a metal, replaces element B, a metal in the compound. Therefore, more reactive metals replace less reactive metals, while reactive non-metals replace less reactive non-metals.
An example is the reaction of Tin chloride and zinc. In this reaction, zinc replaces tin to form zinc chloride and tin as a single element.
SnCl2 =+ Zn = ZnCl 2 + Sn2
5. Acid-Base Reactions/Neutralization
Neutralization reactions are double reactions that occur between acids and bases. For example, an acid-base reaction produces water and salt. An example is when hydrochloric acid reacts with sodium hydroxide, and the products are sodium chloride, salt, and water.
In this example, HCL is the acid, while NaOH is the base. In a practical example, we can have baking soda reacting with vinegar. The products we mostly use when cleaning our homes.
HCL + NaOH = NaCl + H2O
6. Combustion Reactions
These are reactions that involve the burning of compounds. For example, reactants like hydrocarbon react with oxygen to produce carbon dioxide and water. These reactions usually produce energy in the form of heat or light.
CH4 + 2O2 = CO2 +2 H2O
7. Precipitation Reaction
A precipitation reaction is a double replacement reaction and occurs when two soluble salts combine to form an insoluble precipitate.
Iron chloride reacts with sodium hydroxide to form sodium chloride and iron hydroxide.
FeCl3 aq + 3NaOH aq = 3NaCl aq +FeOH3 S/ppt
8. Redox Reaction
These are chemical reactions that involve oxidation and reduction. Oxidation is the addition of oxygen, while reduction is the addition of hydrogen (or removal of oxygen)—for instance, the reaction of copper oxide with hydrogen forming copper metal and water. Copper oxide undergoes reduction by losing oxygen atoms, while hydrogen undergoes oxidation by gaining oxygen atoms.
CuO + H2 = Cu + H2O
Again, you can still define an oxidation-reduction reaction as the change of an oxidation number of a molecule, atom, or ion by gaining or losing an electron. Redox reactions are very common and form the basic functions of life, like photosynthesis, respiration, combustion, and rusting. Therefore, to remember the meaning of oxidation, take it as a loss and reduction as a gain.
The elements that exchange electrons in this reaction are known as the oxidizing and reducing agents. An oxidizing agent is an element that accepts electrons and thus oxidizes the other elements. While a reducing agent, it donates electrons reducing other species in a chemical reaction. For more details on the redox reaction, check this article.
Types of Chemical Reactions Worksheet Answers
We have compiled a list of types of chemical reaction worksheets for most of the common chemistry equations that you may get from your chemistry test or exam. Mostly, your teacher will ask you to identify the 5 types of chemical reactions worksheet answers. However, we have gone beyond and compiled a list of several chemical reactions answer keys as well as balanced equations as shown below:
- Reaction: 2 NaBr+Ca(OH)2→CaBr2+2 NaOH
Type: Double Displacement
Balancing and type are correct. - Reaction: 2 NH3+H2SO4→(NH4)2SO4
Type: Synthesis
Balancing and type are correct. The synthesis reaction here involves the formation of a compound from simpler materials. - Reaction: 4 C5H9O+29 O2→20 CO2+18 H2O
Type: Combustion
Balancing and type are correct. This is a typical combustion reaction where a hydrocarbon combusts in the presence of oxygen to produce carbon dioxide and water. - Reaction: 3 Pb+2 H3PO4→3 H2+Pb3(PO4)2
Type: Single Displacement
Balancing and type are correct. This reaction involves lead displacing hydrogen from phosphoric acid, forming hydrogen gas and lead phosphate. - Reaction: Li3N+3 NH4NO3→3 LiNO3+(NH4)3N
Type: Double Displacement
Balancing and type are correct. This is a double displacement reaction involving the exchange of ions between two compounds. - Reaction: 3 HBr+Al(OH)3→3 H2O+AlBr3
Type: Double Displacement
Balancing and type are correct. In this reaction, hydroxide ions and bromide ions switch places between their respective compounds. - Reaction: Na3PO4+3 KOH→3 NaOH+K3PO4
Type: Double Displacement
Correctly balanced and classified. - Reaction: MgCl2+Li2CO3→MgCO3+2 LiCl
Type: Double Displacement
Correctly balanced and classified. - Reaction: C6H12+9 O2→6 CO2+6 H2O
Type: Combustion
Correctly balanced and classified. - Reaction: Pb+FeSO4→PbSO4+Fe
Type: Single Displacement
Correctly balanced and classified. - Reaction: CaCO3→CaO+CO2
Type: Decomposition
Correctly balanced and classified. - Reaction: P4+3 O2→2 P2O3
Type: Synthesis
Correctly balanced and classified. - Reaction: 2 RbNO3+BeF2→Be(NO3)2+2 RbF
Type: Double Displacement
Correctly balanced and classified. - Reaction: 2 AgNO3+Cu→Cu(NO3)2+2 Ag
Type: Single Displacement
Correctly balanced and classified. - Reaction: C3H6O+4 O2→3 CO2+3 H2O
Type: Combustion
Correctly balanced and classified. - Reaction: 2 C5H5+Fe→Fe(C5H5)2
Type: Synthesis
Correctly balanced and classified. - Reaction: SeCl6+O2→SeO2+3 Cl2
Type: Single Displacement
Correctly balanced and classified. - Reaction: 2 MgI2+Mn(SO3)2→2 MgSO3+MnI4
Type: Double Displacement
Correctly balanced and classified. - Reaction: O3→O+O2
Type: Decomposition
Correctly balanced and classified. - Reaction: 2 NO2→2 O2+N2
Type: Decomposition
Correctly balanced and classified.
Chemical Reactions Answer Key
As a company, we offer various answers key to chemical reactions and other topics in Chemistry as follows:
Writing Equations Answer Key
When writing an equation, the left-hand side represents the reactants while the right products. Therefore, an equation should contain stoichiometric coefficients that show the relative amount of the products and reactants.
For an illustration, check this equation.
A aq + B g= Cs + D l, where A and B are reactants, and CD are products
A stoichiometric coefficient is a number that comes before the atoms, molecules, or ions -for instance, 1 Pb(NO3)2 +2KI = 1Pbl2 +2KNO3; in this equation, 1, 2, 1, and 2 are the stoichiometric coefficients for this equation. These numbers are important to help you in balancing chemical equations.
Also, we should indicate the state symbols while writing chemical equation reactions. The state symbols are as follows.
Aqueous – aq
Gas – g
Liquid – l
Precipitate – ppt ( insoluble)
Solid – s
Chemical Reactions Answer Key
We guide how to identify and write a type of chemical reaction in words and symbols in the types of reactions worksheet below.
| Reaction | Definition | Equation |
| Synthesis | two or more elements combine to form a complex substance | A +B = AB |
| Decomposition | compounds breakdown to form simpler substances | AB =A+B |
| Single replacement | An element replaces another one in a compound | AB+C = AC +B |
| Double replacement | When atoms in different compounds trade for positions | AB+CD= AC+BD |
Examples: Identify the following chemical reactions.
1. Zinc and lead (II) react to form zinc nitrate and lead–word equation
Zn + Pb(NO3)2 = Zn(NO3)2 + Pb- symbols equation, a single replacement reaction that involves zinc and lead metals. In the reactivity series of metals, zinc appears above the lead, thus why it replaces lead nitrate in this example.
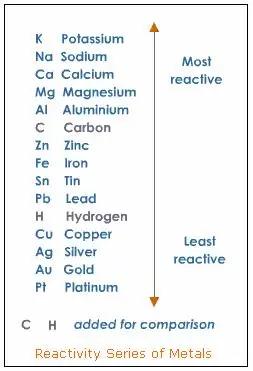
Reactivity Series of Metals Chart
2. 2NaCl aq +F2g = 2 NaFs + Cl2g
The equation represents a single-replacement reaction. Here we have the negatively charged ions, where fluoride replaces chlorine in the compound. Fluoride and chlorine are halogens in the periodic table. Therefore, the halogens at the top usually replace the elements below them.
Balancing Equations Answer Key
We give you solutions for identifying the parts of a chemical equation, describing the equation in words and symbols then balancing.
Look at some of the examples below;
1. Sodium plus water form sodium hydroxide and hydrogen. In symbols, sodium (Na), water (H2O), and oxygen (O2)
Na +H2O = NaOH + O2
To balance the equation the answer will be = 2Na + 2H2O = 2Na(OH) + H2
On the reactants side, we have two atoms of sodium, four atoms of hydrogen, and 2 of oxygen. On the product side, we have two atoms of sodium, two atoms of oxygen, and four atoms of hydrogen, which balances the equation.
2. Aluminum reacts with lead(II) nitrate to give aluminum nitrate and lead metal
2 Al + 3 PB(NO3)2 = 2Al(NO3)3 +3Pb
our equation is complete and balanced. On the reactants side, we have two aluminum atoms, three lead atoms, six nitrogen atoms, and 18 oxygen atoms. We have two aluminum atoms, 6 nitrogen atoms, 18 oxygen atoms, and three lead atoms in the products.
Other Answer Keys That We Offer
- Predicting the products of chemical reactions
- Factors affecting rates of chemical reactions
- Ion concentrations in solutions
- Finding the unpaired electrons
- Calculating molar mass
- The structural formula of alkenes
- Mastering stoichiometry
- Determination oxidation numbers
- Valance electrons and ionic change
- Solubility tests
- Quantum theory
We offer solutions for the above topics and other areas you may have challenges in chemistry. Don't hesitate to reach out and seek answers from our chemistry homework help experts.
Frequently Asked Questions
1. Where can I get Types of Chemical Reactions Worksheet Answers
From homework market chemistry tutors. They will provide accurate answers to all your chemical reaction questions.
2. What are the 5 Types of Chemical Reactions Worksheet?
The 5 types of chemical reactions are:
- Synthesis reaction/combination
- Decomposition reaction
- Single-replacement reaction
- Double replacement reaction
- Combustion reaction
3. Do You Help Balancing Chemical Equations and Types of Reactions Worksheet
Conclusion
Chemical reactions occur everywhere in our environment. Imagine all the activities in the kitchen, industries, and natural environment; they all involve chemical reactions.
The basic chemical reactions are synthesis, decomposition, and single and double replacement reactions also known as double replacement reactions or metathesis. Other reaction types like precipitation and neutralization still fall under double replacement.
The key to understanding chemical reactions is mastering what happens to reactants and products in each type of reaction. When you grasp this concept, it will be easy to classify chemical reactions. Again, ensure you learn all the symbols of elements and how to write balanced chemical reaction equations. If you need help, you can always place an order with us and our chemistry tutors will assist you.
Prices start at $12.99 per page (275 words) for writing and $8.5 for editing and proofreading.
- MONEY BACKGUARANTEED
- NO HIDDENCHARGES













